The GLUTECH Trial explores if gluten detection technology may help improve the treatment of newly diagnosed celiac disease.
This is a multisite trial funded by the National Institutes of Health.
Our Aims
To understand whether gluten detection technology improves intestinal healing.
To understand how gluten detection technology influences adherence to gluten-free diets, celiac disease symptoms, and quality of life.
Who is eligible to participate?
Adults (18 to 75 years old) who have been biopsy-diagnosed with celiac disease within the past 4 months and do not currently use gluten detection technology are eligible to participate, in addition to other criteria.
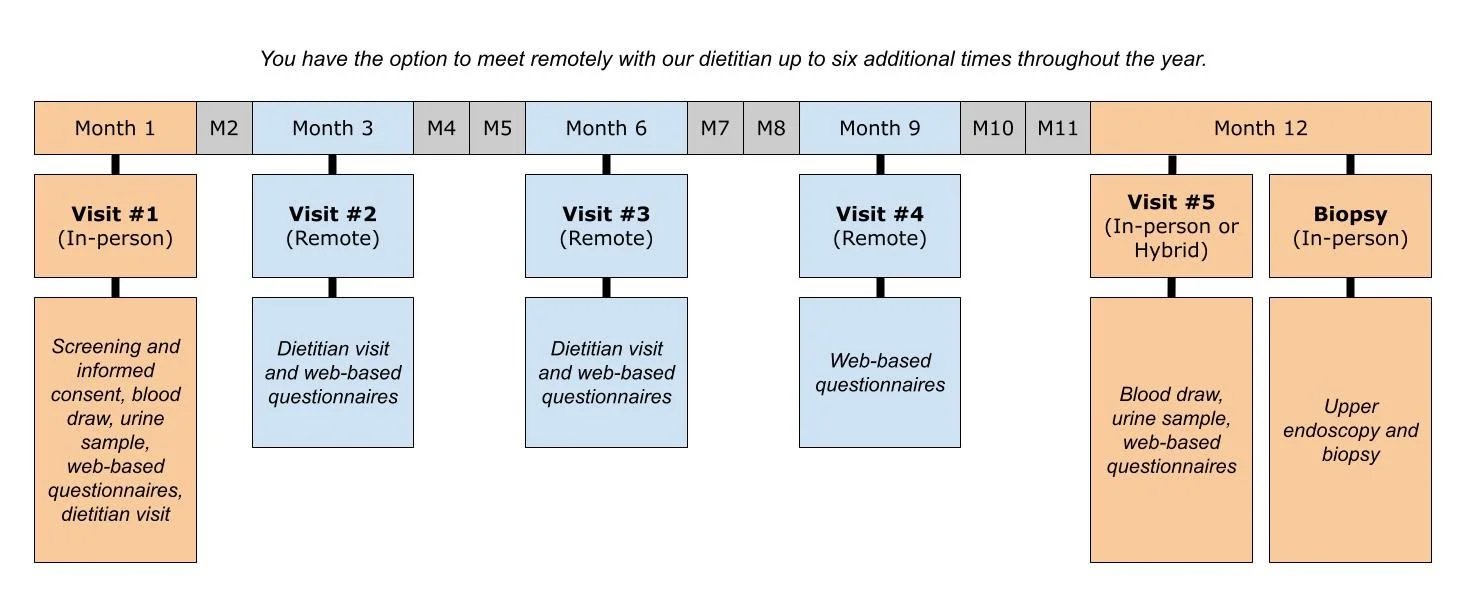
How long does this study last?
The length of this study is 12 months. During this time, you will participate in five health care visits – two are in-person and three are virtual (remote).
What does this study involve?
This study involves being randomly assigned to one of two groups:
The standard of care group involves ongoing visits with a dietitian.
The standard of care & technology group involves ongoing visits with a dietitian and the use of gluten technology.
You have a 50% chance of being in the standard of care group and a 50% chance of being in the standard of care & technology group.
What will I be asked to do?
You will have the following procedures done as part of the study: blood draw, urine sample, endoscopy with biopsy of the small intestine, questionnaires, meetings with the dietitian, and (if you are assigned to the standard-of-care plus technology group) use of gluten detection technology.
Will I be compensated?
Yes. Details about compensation will be provided upon enrollment, before you decide whether to participate.